June 2025 – DOD Requires Common Forms for Biosketches and Current and Pending Support for all upcoming grant applications
The U.S. Department of Defense have updated their application instructions to require the use of Common Forms for Biosketches and Current and Pending Support for all upcoming grant applications.
Common Forms are standardized templates that are created and certified in Science Experts Network Curriculum Vitae (SciENcv). We have curated the information below to provide guidance and resources for those planning to submit in upcoming DoD grant cycles.
NSF currently requires Common Form biosketches and current pending support, but NIH’s adoption of Common Forms, initially slated to begin on May 25, 2025, has been delayed but is anticipated.
April 3, 2025 – Executive Level II Salary Cap Increase
NIH released NOT-OD-25-085 today, confirming an increase in the Executive Level II salary. “The Office of Personnel Management has released new salary levels for the Executive Pay Scale. Effective January 1, 2025, the salary limitation for Executive Level II is $225,700.
For active awards, including awards that have been issued in FY 2025 (continuation and new) that were restricted to Executive Level II, if adequate funds are available, and if the salary cap increase is consistent with the institutional base salary, recipients may rebudget funds to accommodate the current Executive Level II salary level. Recipients may not draw down funds, whether direct or indirect costs, to pay salaries above the salary rate limitation, and recipients must have established policies and procedures that are consistently applied regardless of the source of funds.” Read the full NOT-OD-25-085 notice here.
March 2025 – NIH Common Forms Implementation Postponed
Per the NIH, implementation of the Common Forms for Biosketches and Other Support has been postponed until further notice. https://grants.nih.gov/policy-and-compliance/implementation-of-new-initiatives-and-policies/common-forms-for-biosketch
January 6, 2025 – Community Days Webinar on Updated NIH Security Best Practices for Users of Genomic Controlled-Access Data
In preparation for changes surrounding data management and access practices under the Genomic Data Sharing (GDS) Policy, NIH is hosting two information sessions on Wednesday, January 8, 2025 and Friday, January 10, 2025 from 10:00 AM – 11:00 AM ET.
Read more about the NIH community webinars here.
December 19, 20204 – Implementation Update for NIH Data Management and Access Practices Under the Genomic Data Sharing Policy
As you may have heard, the NIH has published an Implementation Update for Data Management and Access Practices Under the Genomic Data Sharing Policy, effective on January 25th, 2025. This updated policy adds additional data security requirements and applies to all new and competitive NIH applications and agreements that support the following activities:
NIH will treat cloud workspaces meeting the above criteria as controlled-access data repositories subject to the relevant expectations under this update. A list of NIH databases that are subject to these requirements may be viewed here: NIH Security Best Practices for Controlled-Access Data and Repositories | Data Sharing.
Specifically, users of controlled-access data will be expected to follow the NIH Security Best Practices for Users of Controlled-Access Data and attest to implementing an environment that meets the standards of the NIST SP 800-171 Protecting Controlled Unclassified Information in Nonfederal Systems and Organizations to store and use downloaded datasets from these repositories. More references are provided below.
Users and Developers who will access NIH controlled-access repository data will be required to manage the data in a NIST SP 800-171 compliant environment.
To meet this requirement, IT@JH is establishing NIST SP 800-171 security compliance for several institutionally managed research IT environments, including SAFESTOR storage, SAFER desktop, DISCOVERY HPC, and more, which you can leverage at a largely subsidized cost.
Developers must also agree to the Standard Language for Developer Terms of Access in the Terms and Conditions of Award which include a requirement to take the NIH Security Awareness Course.
If after January 25, 2025, you are planning to:
please submit a request using the Research IT Intake Form to review your options with the Research IT team. Please keep in mind lead time in getting your environment set up with the appropriate access and tools. Your JHURA and ORA representatives will be unable to submit an application or a request for data access to NIH without verification of the secure environment.
Please also note that dbGaP data access agreements with renewals prior to January 25th, 2025 will continue to follow current security requirements until the next renewal cycle. Faculty are strongly encouraged to begin the process of security evaluation as soon as possible to ensure there are no delays in renewing.
Please let us know if you have any questions we can help clarify as we support you through this change.
Thank you in advance!
References:
October 11, 2024 – National Science Foundation Mandatory Multifactor Authentication for Research.gov Sign-in Effective on 10/27
The National Science Foundation (NSF) announced that effective October 27, 2024, external users must first complete a one-time Multifactor Authentication (MFA) enrollment process and use the MFA method to sign into Research.gov. Additionally, users must:
More information from the NSF can be found here: Dear Colleague Letter: Multifactor Authentication Implementation for Research.gov (nsf25011) | NSF – U.S. National Science Foundation. The NSF will be releasing training information on October 27, 2024. Questions should be directed to your ORA Grants Associate, while technical questions should be directed to the NSF Helpdesk.
September 25, 2024 – Changes to Data Management and Sharing (DMS) Plan Progress Reporting and the Submission of Revised DMS Plans Are Coming on October 1st
On October 1, NIH is adding several new Data Management and Sharing (DMS) questions to Research Performance Progress Reports (RPPRs) and updating the process for submitting revised DMS Plans to NIH for review. In brief:
An updated NIH RPPR Instruction Guide will be posted to the Research Performance Progress Report (RPPR) page on October 1, 2024. Users should contact the Grants Management Specialist listed on the award’s most recent year’s Notice of Award for questions about RPPRs. For technical issues related to eRA Commons Modules, users should contact the NIH eRA Service Desk.
The NIH announcement can be found here.
August 23, 2024 – New JHU Facilities and Administrative (F&A) Rate Agreement for FY25
JHU has a new F&A rate agreement for FY25. A copy of the current rate agreement, dated July 23, 2024, and tables outlining the new rates can be found on the ORA Indirect Costs and Fringe Benefits Rates page here..
July 31, 2024 – NIH’s Adoption of Common Forms for Biosketches and Other Support by May 25, 2025
NIH confirmed the adoption of Common Forms for Biosketches and Other Support for all applications and progress reports (RPPRs) by May 25, 2025. Read the notice NOT-OD-24-163 here.
A high-level summary of NIH specific updates are as follows:
General Information
Biographical Sketch
Current and Pending (Other) Support
February 13, 2024 – New NIH Guidance on Marking Changes in Resubmission Applications
In a notice released this week, NIH announced that markups should no longer be used to identify changes in Resubmission applications for proposals due on or after May 25, 2024. NIH’s NOT-OD-24-061 states:
“This Notice informs the applicant community that, effective May 25, 2024, this guidance replaces previous guidance on marking changes in Resubmission applications. The use of markups such as bracketing, indenting, highlighting, bolding, italicizing, underlining, margin lines, change in typography, font, or font color, or any other type of markup should not be used to identify changes in Resubmission applications.
Changes made to a Resubmission application should only be outlined in the Introduction attachment. The Introduction must include a summary of substantial additions, deletions, and changes to the application. It must also include a response to weaknesses raised in the Summary Statement. Unless otherwise indicated in the Table of Page Limits, the Introduction may not exceed one page.”
Reminder: NIH’s New Access Requirements Regarding Foreign Subrecipients Goes Into Effect on January 1, 2024
Effective January 1, 2024, all NIH awards will include terms requiring written agreements with all foreign subrecipients requiring access to copies of all lab notebooks, all data and all documentation that supports the research outcomes described in a subrecipient’s progress report submitted to JHU. To ensure compliance with this requirement, the language below will be incorporated into JHU templates as follows:
Outgoing JHU Subagreement (Foreign Subreipients)
By signing this agreement, Subrecipient agrees to provide access as a deliverable to copies of all lab notebooks, all data, and all documentation that supports the research outcomes as described in the progress report, to JHU with a frequency of no less than once per year, in alignment with the timing requirements for Research Performance Progress Report described in Attachment 4. Subrecipient will make such data available via the subrecipient’s secure file sharing or document management platform to the JHU Principal Investigator.
Subrecipient Entity Letter of Intent (Sub Institution Approval for Inclusion of a Sub in a JHU Proposal)
If the Prime Awarding Sponsor is the National Institutes of Health, Subrecipient Institution is aware of, and willing to comply with, the requirements in Section 15.2.1 of the NIH Grants Policy Statement and will provide JHU with access to copies of all lab notebooks, all data, and all documentation that supports the research outcomes as described in the progress report, no less than once per year to coincide with the Research Performance Progress Report (RPPR) submission. Such access may be entirely electronic.
See the SOM Outgoing Subrecipient LOI Template.
Subrecipient PI Letter of Support/Collaboration (Sub PI only for placement in a JHU Proposal)*
I am aware all foreign subrecipients receiving NIH funds are subject to the regulations reflected in NOT-OD-23-133 and NOT-OD-23-182 and should an award be made, our institution must agree to abide by all requirements.
*ORA does not have a template for the Subrecipient PI Letter of Support/Collaboration as this typically originates from the PI. If the PI chooses to include this letter of support/collaboration, please include the language above.
Please note that if the subrecipient is unwilling to agree to the access requirements as outlined here, then a subagreement cannot be issued.
The Principal Investigator will be responsible for accessing and reviewing such lab notebooks, data, and documentation prior to submission of the progress reports to ORA/department signing officials for submission to NIH.
Department and/or post-award staff will receive a direct email from the ORA Outgoing Subawards team (via [email protected]) requesting the submission of a SWiFT record for all subagreements that require modification to include this new requirement.
Please direct any questions regarding this requirement to your department’s assigned ORA Grants Associate and/or Subawards Associate.
ORA Virtual Office Hours – Second Thursdays at 11:00 am
Please join ORA for monthly drop-in virtual Office Hours on the second Thursday of the month at 11:00 am. To review the topic schedule, register for specific sessions, and view prior session slides, please visit the ORA virtual office hours page.
If you would like to submit questions for the Q&A ahead of time, please send them to [email protected] or [email protected] with “Office Hours Q&A” in the email subject. We look forward to hearing from you!
Fibi Updates – Go-Live is October 23rd!
Fibi, the software that will replace JHU’s current proposal preparation, routing, approval and reporting platform (Coeus), will go-live on October 23, 2023. Please visit the ORIS WEBSITE to familiarize yourself with Fibi, practice in the testing environment, and review training material options and opportunities.
September 27, 2023 – NIH Notice #NOT-OD-23-185 – Prior Approval Requests for Revisions to an Approved Data Management and Sharing (DMS) Plan Must be Submitted Using the Prior Approval Module
This NIH notice clarifies the process for submitting DMS revisions requests and requires these requests to be submitted via the eRA Commons Prior Approval module. Additional information on the submission process can be found in NOT-OD-23-185 here. Please work with your ORA Grants Associate to submit a prior approval request.
September 19, 2023 – An Update on the NIH Foreign Subaward/Consortium Policy – Effective 1/1/2024
NIH has issued its Final Updated Policy Guidance for Subaward/Consortium Written Agreements: https://grants.nih.gov/grants/guide/notice-files/NOT-OD-23-182.html
The Policy goes into effect on January 1, 2024 and updates Section 15.2 of the NIH Grants Policy Statement to include the following clarifications:
15.2 ADMINISTRATIVE AND OTHER REQUIREMENTS
The following highlights several areas within the consortium relationship that the recipient needs to address with consortium organizations receiving subawards under a grant to ensure compliance with NIH requirements. The requirement for a written agreement addressing these and other areas is specified in this section. NIH will not support any agreement that does not meet the minimum requirements outlined in the written agreement section below (15.2.1). NIH reserves the right to request copies of the written agreement and relevant supporting documentation as needed, as part of its oversight responsibilities. Failure to provide requested documentation may lead to remedies for noncompliance and potential enforcement actions (see 8.5, Specific award conditions and remedies for noncompliance).
NIH expects recipients to ask potential subrecipients, at the application stage, to submit language in their letters of support indicating their awareness of these requirements and the subrecipient’s willingness to abide by all requirements should an award be issued.
Note that most of these requirements only apply to a recipient’s consortium relationships with sub-recipients. When the relationship is with a vendor that is providing routine goods and services within normal business operations that are ancillary to the operation of the research program, the public policy requirements listed below do not apply. The vendor must also be providing similar goods and services to many different purchasers and provide them in a competitive environment.
15.2.1 Written Agreement
The recipient must enter into a formal written agreement, signed, and agreed to by both parties, with each consortium participant/subrecipient that addresses the negotiated arrangements for meeting the scientific, administrative, financial, and reporting requirements of the grant, including those necessary to ensure compliance with all applicable Federal regulations and policies and facilitate an efficient collaborative venture. If a subrecipient is unwilling to accept the requirements outlined in this section, by signing a written agreement, then an agreement cannot be issued. At a minimum, this agreement must include the following:
Note: All current requirements remain in place, with the addition of:
The NIH has provided FAQ’s on the Policy which are found here: https://grants.nih.gov/faqs#/subawards.htm?anchor=4304
September 6, 2023 – Postdoctoral Fellows Can Serve as PI on Select Proposals
As of September 6, 2023, the Office of Research Administration will accept and submit applications for sponsored projects where Postdoctoral Researchers serve in the role of Principal Investigator where the following criteria are met:
Please contact your ORA Grants or Contracts team member with any questions.
July 31, 2023 – NIH Updates Budget Form Data Management and Sharing Costs Guidance for Proposals Due on or after October 5, 2023
NOT-OD-23-161: Effective for applications submitted for due dates on or after October 5, 2023, NIH will no longer require the use of the single DMS cost line item in the R&R Budget. DMS costs must be requested in the appropriate cost category, e.g., personnel, equipment, supplies, and other expenses, following the instructions for the R&R Budget Form or PHS 398 Modular Budget Form, as applicable. While the single cost line item is no longer required, NIH will require applicants to specify estimated DMS cost details within the “Budget Justification” attachment of the R&R Budget Form or “Additional Narrative Justification” attachment of the PHS 398 Modular Budget Form, pursuant to the instructions.
June 21, 2023 – Updates on the Federal Demonstration Partnership (FDP) NIH Data Management and Sharing Plan Template Pilot Program
Dear Colleagues,
In April 2023, we announced that Johns Hopkins University is participating in the Federal Demonstration Partnership (FDP) NIH DMSP Template Pilot, which is testing the effectiveness and usability of two DMSP templates developed in collaboration with representatives from FDP, including participating NIH Institutes and Centers (ICs). As a reminder, two templates are being tested and investigators are free to choose which one is most suitable for the nature of their work:
Both templates have been approved for use by NIH and have been specifically designed to increase the likelihood that your DMSP will provide all the information the IC/program requires in order to approve the DMSP rather than needing to request changes at the Just-In-Time (JIT phase).
NIH-FDP Templates Now Available in the DMPTool
JHU has been recommending the use of a free online application called DMPTool. We are happy to announce that you can now use DMPTool to draft your plan using the one of two NIH-FDP Templates.
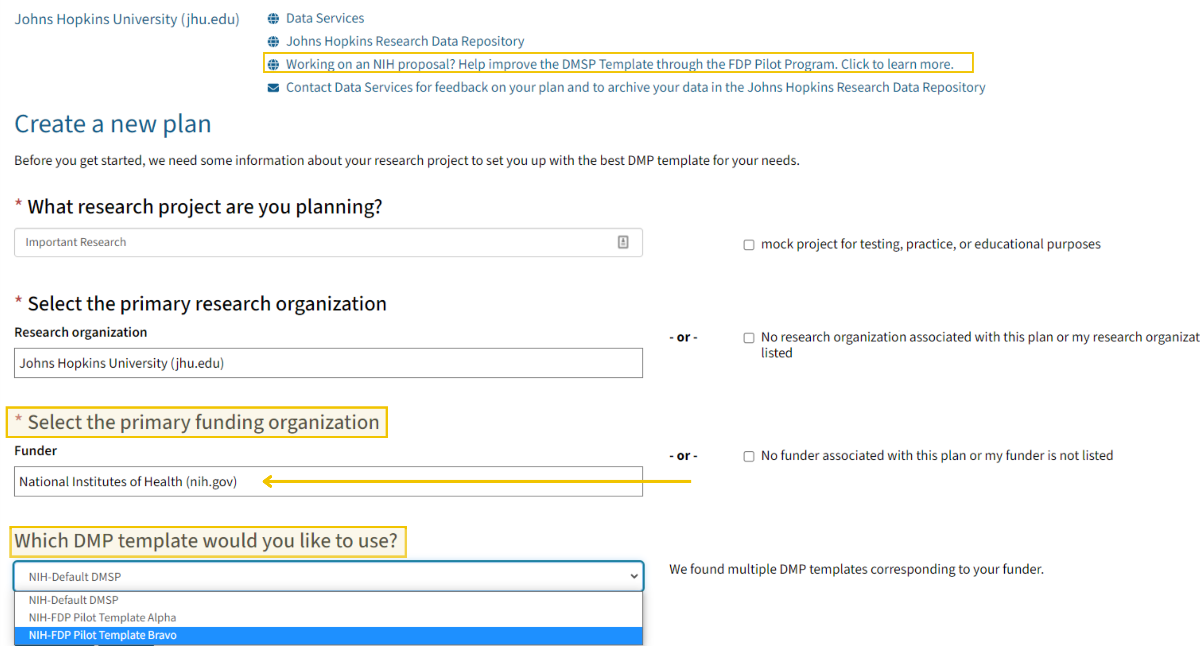
How to Select an FDP template in the DMPTool
You can hit the “request feedback” button within the DMPTool to get feedback from Data Services, who will review your Plan and provide feedback in a timely manner.
New Coeus Questions:
When creating an NIH Proposal in Coeus, you will now see updates in Coeus to include questions regarding the use of the two FDP templates. Please be sure to check yes if you have used the templates so that we can keep track of the testing.
Use a NIH-FDP Template for upcoming NIH Deadlines (July 5)
The ultimate goals of the pilot are to harmonize DMSP requirements across NIH ICs and programs and to mitigate the administrative burden for researchers associated with DMSP development and implementation. Please consider using an NIH-FDP Template for your upcoming grant proposal deadlines!
More details on the pilot can be found on the JHURA Website. Also, JHURA/ORA, Data Services, and Welch Medical Library each have online resources to help you navigate the requirements of the new NIH Policy,
Please let us know if you have any questions or concerns. We truly hope you will add your voice to this important opportunity to shape the development of future NIH-required formats!
Sincerely,
Alexandra Albinak
Associate Vice Provost for Research Administration
Johns Hopkins University
Thomas Burns
Associate Dean for Research Affairs
Office of Research Administration
FAQs
What will be done with our feedback?
The feedback received will enable NIH to adopt a single format at the conclusion of the pilot (as opposed to multiple formats for each IC) which will lessen the future administrative burden.
Why is it important to have one format?
The adoption of a single format will allow us to most effectively use the DMP Tool. FDP anticipates having both templates available for use with the DMPTool by June.
January 25, 2023 – JHU Data Services Data Management and Sharing Plan webinars
WRITING YOUR NIH DATA MANAGEMENT AND SHARING PLAN
Date: Wednesday, January 25, 2023
Time:12:00pm – 1:00pm
Registration link: https://jhu.libcal.com/event/10151186
WRITING DATA MANAGEMENT PLANS WITH DMPTOOL
Date: Wednesday, January 25, 2023
Time: 1:30pm – 2:30pm
Registration link: https://jhu.libcal.com/event/10151128
January 10 & 19, 2023 – ORA Q&A Sessions with Data Services & Welch Library
ORA hosted two joint NIH Data Management and Sharing Policy webinars with Data Services and Welch Library to discuss the new NIH policy and review available resources for Data Management and Sharing Plan drafting and budgeting. The webinar recording can be viewed here and the presentation slides can be downloaded here.
Additional resources referenced in the webinar include the Data Services flyer, as well as The Welch Library’s Data Management & Sharing Planning Guide.
Visit the JHU webpage on the new NIH Data Management and Sharing Policy for additional resource links.
December 14, 2022 – JHURA Brown Bag Data Management and Sharing Policy webinar
December 2022 – New NIH Policy: Data Management & Sharing Requirement for NIH applications effective January 25, 2023
All NIH applications due on or after January 25, 2023 that will result in the generation of scientific data will be required to include a Data Management and Sharing Plan. Under this new Data Management and Sharing (DMS) policy, NIH expects that investigators and institutions:
Additional information about planning & budgeting, submission & review, and implementation can be found on the NIH Data Management and Sharing Policy website.
The JHU Data Services website is an excellent internal resource for further guidance on research data management and sharing: